First Law of Thermodynamics | Definition, Eqation, Examples

Hello, how are you guys? I hope you all are very fine and read our articles on a daily basis. today we present a very interesting topic of thermodynamics – The first law of thermodynamics. thermodynamics branch of Physics in which we read about internal energy, heat exchange, work done by the system or on the system, and many other important things. there are some laws of thermodynamics. In this article, we will tell you about the first law. we will also tell you about the internal energy of any system. if you want to know about thermodynamics in detail, you can go through our previous article by the link given below. Let’s move on to our article.
From the exam point of view, there are described very important questions asked a number of times and different types of examination. Such types of questions are like the law of thermodynamics first law, the first law of thermodynamics in physics, the application of the first law of Thermodynamics, the example of the first law of thermodynamics, what is internal energy, etc. So, please read these questions very carefully. very we will try to make you understand this topic in very simple words.
What is the first law of thermodynamics?
Thermodynamic states in the equilibrium possess different types of state variables one of which is known as internal energy. if we talk about that change in internal energy between the two systems it is equal to the difference in the transfer of heat into the system and the work that is done by the system.
According to the first law of thermodynamics, it is stated that the energy of the universe remains constant always. energy can make itself exchanged between the surroundings and also the system. the most important thing about energy is that it cannot be created nor destroyed. We can say that the first law of thermodynamics states about changing energy by the means of work done in heat transfer.
In very bold lines the first law of thermodynamics States, that heat new is present in the form of energy and thermodynamic processes are subject to the principle of energy conservation. heat cannot be created or cannot be destroyed. Energy can change its form from one form to the other form.
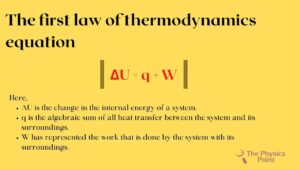
The first law of thermodynamics equation
The equation of the first law of thermodynamics is given below-
ΔU = q + W
Here,
- ΔU is the change in the internal energy of a system.
- q is the algebraic sum of all heat transfer between the system and its surroundings.
- W has represented the work that is done by the system with its surroundings.
Limitations of the first law of thermodynamics
There are some limitations to this law of thermodynamics. This law states that at a time a system in any thermodynamical process always contains certain types of energy balance. The limitation is that this law is unable to explain the feasibility of the process or the changing of the state in which the system undergoes.
Thermodynamics 1st law is also unable to explain why the heat flows from the hot (high temperature) to the cold (low temperature) end. it can only explain the quantity of energy transfer during the thermodynamic process.
First law implementation for the closed system
When we talk about the work done in Thermodynamics for a closed system, it is equal to the multiplication of the pressure applied and the volume change of the system. then the formula of the work done at constant pressure is given below-
W = − P ΔV
Here P represents the constant external pressure acting on the system. ΔV represents the change in the volume of the system. So easily, we can calculate the work done by the above formula.
Examples of the first law of thermodynamics
Here are some common examples of the first law:
- The most common example of this law, is that when you are rubbing your hand the Kinetic motion (Kinetic Energy) of your hands is converted into heat energy.
- The melting of the ice cube is a good example of this law 1 of thermodynamics.
- The electric heater also follows the first law of thermodynamics. because in the heater, the electrical energy is converted into heat energy by doing some work.
What is Internal Energy?
In Thermodynamics the internal energy of any system is represented as ΔE or the ΔE. internal energy is a state function and defines the energy of the molecule or any substance due to its geometry or internal bondings and without having the effect of any external factor such as magnetic field electric field capillarity etc. The energy of any system can be increased or decreased totally depending on the work interaction with its surroundings. The internal energy of any system is increased if the work is done on the system. The internal energy of the system is decreased if the work is done by the system.
One more factor that influences the changing of the enter energy of any system is heat exchange. but the total change in the internal energy of the system is forever zero. for example, if some amount of energy is lost by the system then this energy is absorbed by the surroundings and if some amount of energy is absorbed by the system then this absorbed energy is released by the surroundings. in this way, the total energy remains constant. We can represent this in the way –
ΔU of the system = −ΔU of surroundings
Conclusion
Guys in today’s article we talked about the first law of thermodynamics. we describe your very important types of questions such as what the first law of thermodynamics is, the first law of thermodynamics examples, the first law of thermodynamics equation, etc. To ask any question related to this article you can contact us through the comment section box. You can also give your suggestions regarding this topic. We will meet the next day with the new topic of Physics. Thank you!
